ISO 11040 は、プレフィルドシリンジの設計および機能特性に対応する試験規格です。ISO 11040は、主に製薬業界で使用されており、シリンジ機能が臨床現場で適切に発揮されることを確認するうえで重要となる規格といえます。ISO 11040に基づいた試験評価は、製造工程全体で実行されます。こうした試験の目的は、サブコンポーネントの不良が医師または患者に大きな影響を及ぼす可能性があることから、欠陥のあるデバイスが工場から出荷される可能性を最小限に抑えることにあります。不適切なシールは、薬剤の酸化を引き起こし、製品の貯蔵寿命に影響を与えるおそれがあります。また、シリンジバレルの構造的欠陥はデバイスの不良につながる可能性があります。注記:シリンジを製造する試験室は、 21 CFR Part 11に準拠する必要があります。
規格の説明
ISO 11040は、8項目から構成されています。
- Part1〜3は、歯科環境で局所麻酔薬に使用されるカートリッジのサブコンポーネントに対応します。
- Part 4〜6は、ガラスバレル、プラスチックバレル、プランジャーストッパーなどのプレフィルドシリンジのサブコンポーネントに対応します。
- Part 7は、滅菌済みの充填および仕上げ用途に通常使用される包装システムにに対応します。「充填と仕上げ」とは、最終使用向けシリンジの準備工程であり、サプライチェーンにおいて障害となることがよくあります。
- Part 8には、個々のサブコンポーネントではなく、シリンジ完成品の試験方法と評価基準が記載されます。これらの各試験では、シリンジバレルの耐久性、デバイスの機能的使用(緩み・滑走力、針の貫通力など)、デバイスの閉鎖性および安全機能などを評価します。Part 4およびPart 6に記載される試験方法は、その多くがPart 8での試験方法と類似しています。
自動化の選択肢
ISO 11040のAnnex G.5は、メス型ルアーロック接続部と組み合わせて使用されるリジッドチップキャップの取り外しトルクに特化した評価をするための規格です。 協働ロボット は、ねじり試験対応の万能材料試験機と併用することで、試験室の生産性にきわめて有益となります。基本的にピックアンドプレース操作として機能し、オペレーターへの影響を軽減します。自動化による試験片挿入の再現性は、データのばらつきに大きく影響を及ぼすデバイスのアライメントを改善する上でも理想的といえます。プレフィルドシリンジにはさまざまな容量があるため、サンプルラックは各デバイス形状に対応してカスタマイズできるほか、Bluehill Universal とのシームレスな統合により、新型デバイス向けの試験メソッドを迅速に設定することができます。
最高クラスの6800シリーズ試験機のカタログ
インストロン6800シリーズ万能材料試験機は、他に類のない精度と信頼性を提供します。特許申請中のオペレーター保護機能に基づき、最新のスマートクローズエアキットおよび衝突緩和機能を搭載した6800シリーズは、材料試験をかつてないほどシンプルに、スマートに、安全にします。
Bluehill Universalのカタログ
Bluehill Universalソフトウェアは、タッチ操作と直感的なユーザーエクスペリエンスを念頭に構築されています。標準装備の試験メソッド、数秒で行われるQuickTest、強化されたデータエクスポート、そしてサービスとの直接通信を提供する新機能Instron Connectなどの機能が、これまでよりもシンプルでスマートな試験を可能にします。Bluehill 2やBluehill 3などの旧バージョンソフトウェアからは、簡単に最新バージョンのBluehillにアップグレードできます。
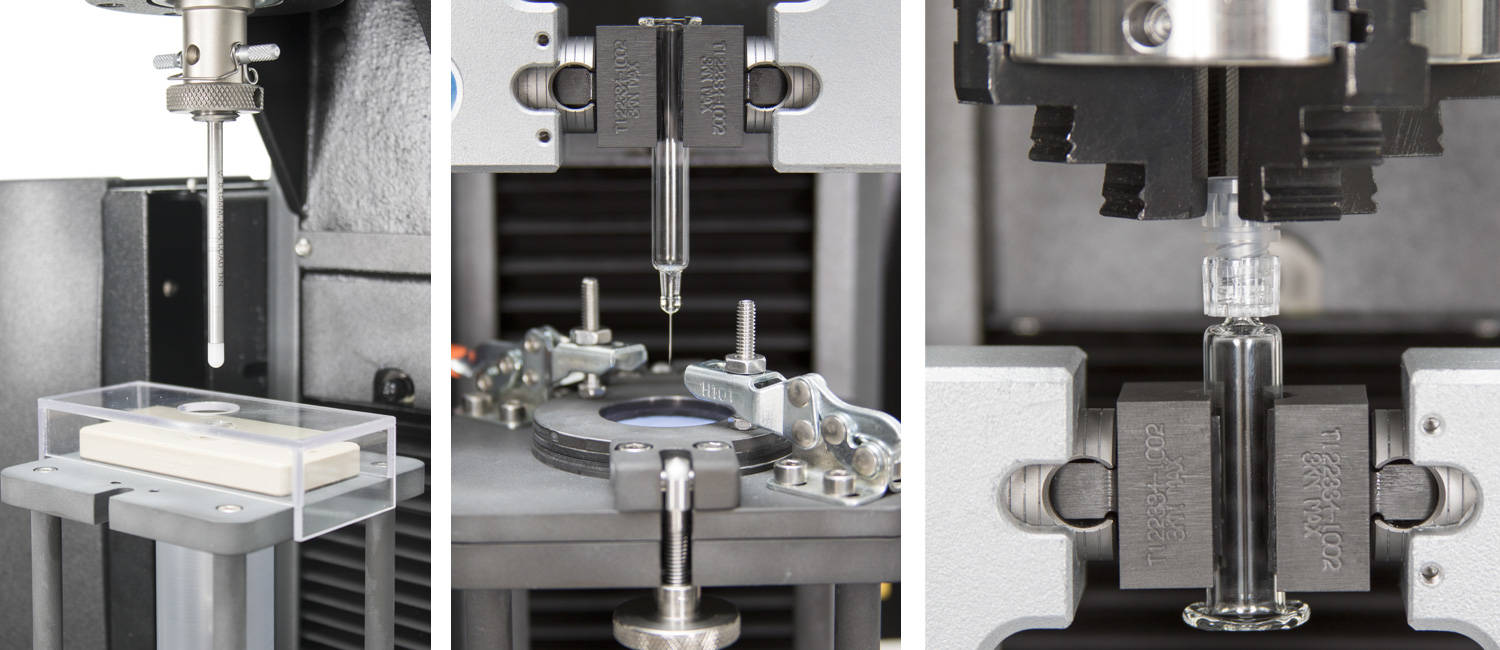
ISO 11040:薬剤充填済みガラス製注射器用の試験治具
インストロンは、ISO 11040の10種の附属書すべてに沿って試験を実施できるように設計された、モジュール式の治具を提供しています。
Torsion Add-On 3.0
Torsion Add-On 3.0は、軸方向およびねじり試験の両方を必要とする医療機器、電子機器、消費者製品、包装、自動車部品などの性能を測定するための簡素なソリューションを提供します。